A causative gene for a highly common type of hearing loss (sensorineural hearing loss, or SNHL) has been identified by a group of Japanese researchers, who successfully replicated the condition using a transgenic mouse. This discovery could potentially be used to develop new treatments for hearing loss. The findings were published on October 5 in the online version of EMBO Molecular Medicine.
The research group included Associate Professor Takehiko UEYAMA (Kobe University Biosignal Research Center, Japan) and Research Fellow Shin-ichiro KITAJIRI (Kyoto University Department of Otolaryngology, Head and Neck Surgery, Japan).
One infant in every 1000 is diagnosed with sensorineural hearing loss (1), making it an extremely common hereditary disease. (For comparison, congenital hypothyroidism is also screened for at birth and affects one in every 3000-5000 Japanese people.) It is also estimated that 25-40% of over-65s suffer from acquired sensorineural hearing loss (commonly known as age-related hearing loss), amounting to as many as 10-15 million Japanese people.
Despite this, treatment development for sensorineural hearing loss is not making progress. This is because the inner ear is a delicate and complex sensory organ that is difficult to research in vitro (outside a living organism). Currently there is no basic cure, and using a hearing aid is still the most effective treatment.
In previous research, scientists discovered about 100 causative genes for sensorineural hearing loss. However, there are many unexplained aspects to the process, such as the type of mutation occurring in these genes, and how this causes hearing impairment. This time the research team identified the causative gene for autosomal dominant nonsyndromic sensorineural deafness, DFNA1. The causative gene for this disease was first suggested in 1997, but doubts were cast regarding its universality and properties.
The research group carried out exon analysis using next generation sequencing (2,3), targeting 1120 Japanese patients suffering from hearing impairments of unknown causes. In two families they discovered a novel mutation in the genetic make-up of DIA1 molecule (DIAPH1), which is involved in the lengthening of linear actin filaments (4). These filaments play an important function in the formation and maintenance of auditory hair and inner ear hair cells. The researchers used biochemical and biological analysis methods on a molecular level to prove that the DIA1 mutant protein created by the mutation is an active form variant that lengthens actin filaments even without external stimulation.

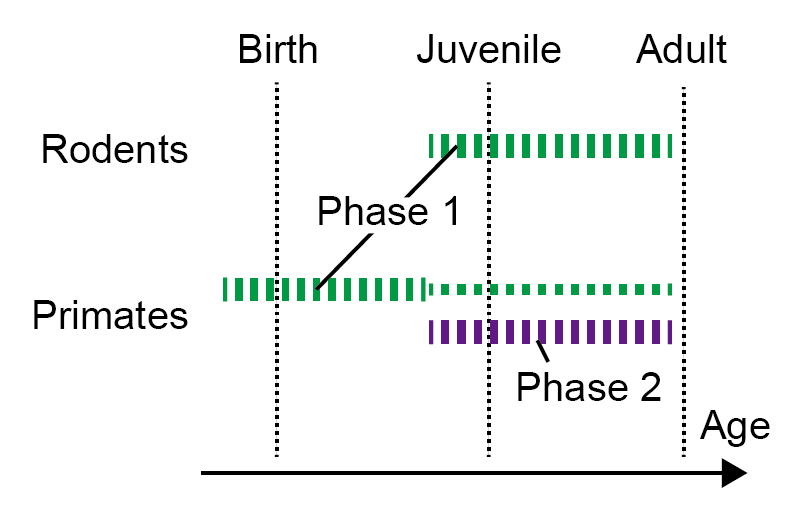
The team also engineered a transgenic mouse that manifests this DIA1 mutant protein. They confirmed that the mouse exhibits traits of sensorineural hearing loss, saying that it “demonstrated progressive deafness, starting in the upper registers when young, and advancing with age until it covered all registers”.
Surprisingly, roughly one third of the causative genes for sensorineural hearing loss discovered so far are genes that encode proteins with functions related to actin, just like the gene identified this time. This means that as many as one third of sensorineural hearing loss cases are related to actin. By using the model transgenic mouse to find the compounds that transform actin functions within the inner ear hair cells, scientists could potentially develop new treatment for other strains of hereditary sensorineural hearing loss in addition to DFNA1. The hearing-impaired mouse could also be a key to discovering treatment for acquired sensorineural hearing loss.

At 5 weeks there is no difference between TG mice and control mice, but at 10 and 25 weeks drop off in the outer hair cells (red circles) and inner hair cells (white circles) is advancing significantly.

In transgenic mice (TG) the auditory hair at the apex of hair cells shows various abnormalities: long parts (arrows), shorter parts (arrowheads), and adhesions in the base.
Technical terms
- 1 Sensorineural hearing loss
- a hearing disorder caused by pathology between the inner ear and the auditory center in the brain
- 2 Next-generation sequencing
- A sequencer that uses a completely difference basis from conventional methods to rapidly determine DNA base sequences
- 3 Exon analysis
- a method that exhaustively analyzes the exon sequence of genomes specifically (the sequence that is transcribed to mRNA)
- 4 Actin filaments
- Part of the cytoskeleton that determines cell shape, these are protein compounds consisting of bundles of actin molecules twisted together in filaments (fibrous structures)
Journal information
- Title
- “ Constitutive activation of DIA1 (DIAPH1) via C-terminal truncation causes human sensorineural hearing loss ”
- DOI
- 10.15252/emmm.201606609
- Authors
- Takehiko Ueyama, Yuzuru Ninoyu, Shin-ya Nishio, Takushi Miyoshi, Hiroko Torii, Koji Nishimura, Kazuma Sugahara, Hideaki Sakata, Dean Thumkeo, Hirofumi Sakaguchi, Naoki Watanabe, Shin-ichi Usami, Naoaki Saito, Shin-ichiro Kitajiri
- Journal
- EMBO Molecular Medicine